In the fast-paced world of life sciences, intellectual property (IP) litigation is a high-stakes arena where scientific complexity meets legal rigor. Navigating these cases, especially in the context of ANDA litigation, requires not only legal acumen but also a deep understanding of drug development, biotechnology, and healthcare technologies.
This is where Patexia Connect steps in, bridging the gap between legal teams and industry experts to provide the critical insights needed to tackle IP challenges head-on. With Patexia Connect, firms are equipped with a network of specialists who ensure that every scientific and regulatory angle is covered, helping them handle even the most intricate cases with confidence.
The High Stakes of ANDA Litigation
Abbreviated New Drug Application (ANDA) litigation is a cornerstone of pharmaceutical IP law, determining whether generic drugs can enter the market. This litigation directly influences drug pricing, innovation, and competition, making it a critical issue for healthcare companies.
As highlighted in our CEO Pedram Sameni’s recent Law360 article, “Takeaways From Novo Nordisk’s Fight For Market Exclusivity”, defending exclusivity in the face of generic challenges is no small feat; in Novo Nordisk’s case, over $90 billion is on the line if patents were to be invalidated. The outcomes of ANDA cases shape the competitive landscape for brand-name and generic drug manufacturers alike.
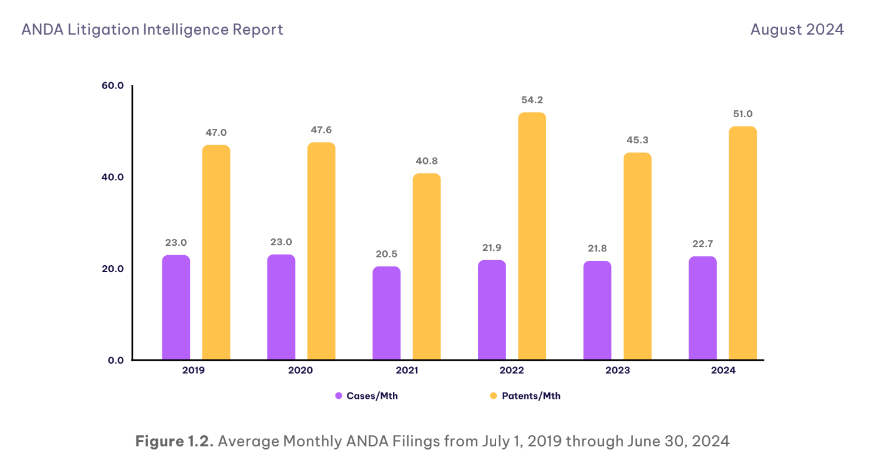
As illustrated in the chart above, our latest ANDA Litigation Intelligence Report report highlights a resurgence in ANDA filings, with the rate increasing to 22.7 cases per month in 2024, compared to 20.5 in 2021. This rise suggests that ANDA litigation is becoming more intense, driven by increasingly complex patent disputes, which require the high involvement of technical experts.
The Pivotal Role of Expert Witnesses
In life sciences litigation, complex technical disputes can make or break a case. Issues of patent validity, infringement claims, and regulatory compliance require expert witnesses who can break down these technical matters and present them clearly to judges and juries.
Patexia Connect ensures that legal teams are paired with the right experts who can simplify complex topics, strengthen legal arguments, and clarify scientific details.
Our experts offer support in key areas:
- Case Preparation and Testimony: Experts contribute invaluable insights during depositions and hearings, helping to clarify technical matters and support robust legal arguments.
- Regulatory and Technical Analysis: Experts evaluate compliance with FDA standards and other regulatory frameworks, aligning legal strategies with industry best practices.
- Product Development and Market Insights: From analyzing competitive landscapes to evaluating technological innovations, experts provide insights that support claims of patent validity, infringement, or exclusivity.
Specialized Expertise Across Life Sciences
Patexia Connect’s network of experts reflects its deep focus on key areas within life sciences:
Biotechnology
Genetic engineering and cell therapies are at the forefront of biotechnology, making this sector a critical area for IP litigation. Patexia’s experts in molecular biology and genomics provide the necessary expertise for defending or challenging patents in this rapidly evolving field.
Among our experts is a biomedical engineer with extensive R&D experience in diagnostics and medical devices who has worked with Fortune 500 companies and startups to advance healthcare Technologies.
Pharmaceutical
With the rise of ANDA litigation, expertise in drug formulation, bioequivalence, and pharmacokinetics is indispensable. Patexia’s network includes seasoned professionals who understand the intricacies of pharmaceutical patents and regulatory compliance.
One of our experts brings over 20 years of experience in drug formulation, including nano-formulation biopharmaceutics, and a distinguished academic background in cardiovascular and cancer pharmacotherapy.
Medical Devices
The increasing integration of digital solutions into medical devices has amplified their complexity. Patexia’s experts bring experience in diagnostics, wearable technology, and surgical instruments, providing both technical and regulatory insights.
For instance, a clinical specialist brought advanced medical knowledge in a healthcare patent litigation case, supporting the legal team in preparing detailed reports and depositions. This expert’s contributions were pivotal in achieving a favorable settlement, showcasing the importance of expert testimony in high-stakes cases.
Why Life Sciences Partners Choose Patexia Connect
For law firms and companies navigating the complexities of life sciences IP law, Patexia Connect provides a strategic advantage. Its curated network of experts combines technical depth with decades of practical experience, ensuring clients receive specialized support tailored to their unique case needs.
By streamlining contracting, facilitating responsive communication, and managing administrative tasks, Patexia enables experts to focus on delivering high-value insights. This seamless collaboration allows legal teams to build more robust cases and achieve better outcomes for their clients.
Discover how Patexia Connect can help you build a stronger, data-backed legal strategy. Contact us to learn more about our services and the experts available to support your cases in the life sciences domain.
